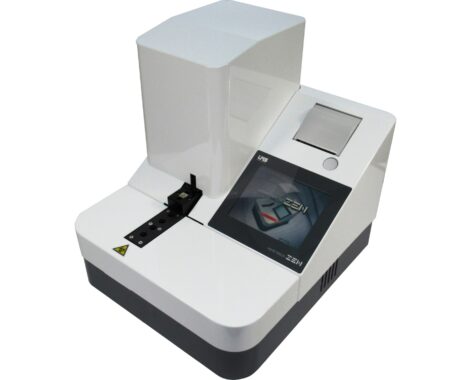
Platelet aggregation assay system; Hematracer Zen
For monitoring of antiplatelet drugs! (Aspirin, Clopidogrel, etc.)
Whole blood can be tested as is (no need for centrifugation)
Sample volume 0.8mℓ/1test
No influence of chyle or blood cell contamination
Compact size includes in the touch panel and printer
Easy maintenance
Specifications
| Product Description | The platelet aggregation is a test to check the aggregation ability (hemostatic ability) of platelets. If the platelet aggregation ability is too strong, clots tend to form and it is necessary to be careful of thrombotic diseases such as myocardial infarction and cerebral infarction. If the platelet aggregation ability is too strong, a thrombus can easily form, and it is necessary to be careful about thrombotic diseases such as myocardial infarction and cerebral infarction. If the platelet aggregation ability is too strong, a thrombus can easily form, and you need to be careful about thrombotic diseases such as myocardial infarction and cerebral infarction. On the other hand, if the aggregation ability is too weak, caution is needed in case of bleeding. Currently, in addition to determining platelet function, this test is also used to check the amount and type of drugs used in antiplatelet therapy (administration of antiplatelet drugs) for the treatment and prevention of cerebral infarction and myocardial infarction. |
|---|---|
| Specifications | Principle: SFP variation method (narrow mouth suction pressure detection method) Filter size: 30μm x 30μm, 300 ports / 1.0 mm diameter Test items: ADP(Adenosine diphosphate), collagen, etc. Samples: Whole blood or PRP (platelet rich plasma) with anticoagulant sodium citrate) Required Sample Volume: Approx. 1 ml for one test Measurement time: Approx. 7 minutes Build-in: Touch panel and printer, USB, RS232C Options: Reagent cooler and Barcode reader Dimensions: 315(W)×360(D)×340(H)mm Weight: 13.5kg Power supply: AC100V 50/60Hz, Power consumption: 65VA Medical device registration number 13B3X00181000004 Class classification General medical device (Class I) Compliant with IEC61010-1(JIS C 1010-1) , IEC61326-2-6 |


